Authors:
Evren Yasar1,2, Jaspal R Singh2*, John Hill3 and Venu Akuthota2
1Gulhane Military Medical Academy, Rehabilitation Center, Interventional Pain Unit, Ankara / Turkey
2University of Colorado, Department of Physical Medicine and Rehabilitation, USA
3University of Colorado, Department of Family Medicine, Division of Sports Medicine, USA
Received: 02 July, 2014; Accepted: 13 September, 2014; Published: 15 September, 2014
Jaspal Singh, MD, Department of Physical Medicine and Rehabilitation, 525 E 68th Street, Baker 16 Floor, NYC, NY 10065, USA, Email:
Yasar E, Singh JR, Hill J, Akuthota V (2014) Image-Guided Injections of the Hip. J Nov Physiother Phys Rehabil 1(1): 039-048. DOI: 10.17352/2455-5487.000008
© 2014 Yasar E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Hip Injections; Ultrasound; Fluoroscopy; Sports medicine
The authors present a technique paper on the utilization of both ultrasound and fluoroscopy guidance for injections about the hip joint. This review draws from specialists including physiatry, family medicine and orthopaedic surgery. We hope the editors and reviewers find this document beneficial to the readership, especially those practicing musculoskeletal medicine and may use this information when performing hip injections.
Introduction
The primary purpose of this technique report is to outline ultrasound- and fluoroscopic-guided office based injections around the hip joint.In addition, this report could serve a reference to physician practicing interventional musculoskeletal medicine.
Traditional causes of hip pain may be divided into either intra-articular or extra-articular sources. The hip joint, pelvis and pelvic girdle muscles have a complex anatomical relationship with proximal and distal structures including the lumbosacral spine and the knee joint. Abnormalities in these regions may cause various referral patterns often mimicking primary hip pain [1]. The character and location of pain is a key element in the differential diagnosis of hip pain. For instance, anterior hip or groin pain often suggests primary involvement of the hip joint itself. However, in a study conducted by Lesher and colleagues, hip joint intra-articular injections were shown to cause referred pain into the buttock (71%), thigh (57%) and groin (55%) [2]. Lateral hip pain that is aggravated by direct pressure is the classic presentation of trochanteric bursitis. Referred pain from facet and sacroiliac joints as well as proximal lumbar radicular pain may also present with pain in the groin or hip. This pain typically originates at the waistline or posterior gluteal areas. The source of hip pain can be further defined by a detailed history and physical examination. However, a portion of hip pain patients may require additional diagnostic work up to further elucidate the etiology.
Radiographic examinations such as plain x-ray, radionuclide scans, magnetic resonance imaging (MRI), computerized tomography (CT) or ultrasonography are useful in the investigation of the possible causes of pain around hip. In addition, several injection techniques, often performed under fluoroscopy or ultrasound guidance, are also helpful to distinguish the conditions causing hip pain. These injection techniques may be used for therapeutic purposes, functional demonstrations, or even joint aspiration. Ultrasound provides real-time radiographic imaging of the musculoskeletal system and in particular has the ability to make dynamic assessments of deep-seated joints, muscles and tendons [3]. Although it is highly operator-dependent, dynamic evaluation of hip girdle tendons and muscles is made possible with utilization of ultrasound.
Increasingly, differential injections of structures about the hip joint are used to guide clinical decision making.For instance, intra-articular injections with positive response to anesthetic are often mandated by surgeons considering operations of the hip labrum [4]. Another common scenario is the patient with hip region pain folowing hip arthroplasty.Ultrasound guided injections can serve as a valuable tool in identifying the source of pain in these subjects [5].
Injectate options
Injection of local anesthetics provides a potential block of nociceptive structures, thus becoming a diagnostic tool with hip pain [6]. These blocks can be performed to confirm the diagnosis of hip pain related to osteoarthritis, bursitis, meralgia paresthetica, lumbosacral roots, lumbar facet or sacroiliac dysfunctions [7,8]. If pain is significantly diminished within minutes after the local anesthetic injection, intra-articular sources vs. extra-articular sources of hip pain can be distinguished [9-11]. The duration of action, the maximum suggested dosage, and the contraindications regarding particular local anesthetics must be considered prior to their use. Local anesthetics act by reversibly inhibiting sodium-specific ion channels on neuronal cell membranes which thereby prevents the propagation of an action potential in the neuron, thus inhibiting pain signal conduction [12] (Table 1).
Cardiac as well as central nervous system toxicites are the most well-known adverse events following inadvertent intravascular or intrathecal injection of local anesthetics [13,14]. Furthermore, local anesthetics can cause a dose-dependent and clinically relevant toxicity to skeletal muscle. Clinical features including muscle weakness and decreased function have been reported after repetitive muscle infiltration of bupivacaine [15-17]. Chondrotoxicity induced by local anesthetics has come into light recently and care must be taken to avoid this complication. Chu and colleagues have performed multiple studies demonstrating that both bupivacaine and lidocaine cause both dose and time dependent cytotoxic effect on bovine as well as human articular cartilage [18-20]. These dose dependencies are most noted with 0.5% bupivacaine and 2% lidocaine while the less potent doses have minimal effect on chondrolysis [21].
Additives to injectate
A range of additives can be used as adjuncts to commonly performed nerve blocks. Sodium bicarbonate can speed up the onset of local anesthesia while epinephrine has been shown to prolong nerve blockade. While tramadol, magnesium, dexmedetomidine, ketamine and other agents have been used, these are not routinely implemented due to the paucity of safety and efficacy data. A discussion of these additives is beyond the scope of this technique paper [22].
Intra-articular Hip Injection
Indications
Hip osteoarthritis (OA) is a common cause of disability and impaired quality of life in older individuals [23] and has a mean prevalence of 8% in the general adult population [24]. Although the primary change is loss of hyaline articular cartilage, secondary changes include osteophyte formation, bony remodelling and changes of the synovium, capsule, ligaments and muscles.Intraarticular hip injections of local anesthetic with or without steroids can determine if the hip pathology is the primary pain generator causing hip pain [25]. Complications from these intraarticular hip injections include joint infection and hemarthrosis, however stict adherence to asceptic technique and the use of image guidance can minimize these [26]. Rarely, air embolism or mild pain and swelling at the injection site can occur. Accordingly, we suggest that single dose vials of sterile medications and contrast be used. Sterile technique is paramount in all interventional procedures. The hip joint has a volume capacity ranging from 8-20 cc [27] and therefore in an effort not to increase intra-articular pressures which could limit functional range of motion, authors suggest injecting a total volume of 8cc (6cc of local anesthetic and 2cc of corticosteroid) [28,29].
As with most injections described in this manuscript, contraindications to injections, esepcially with steroid include, bleeding/clotting disorrders, systemic infection, uncontrolled diabetes and other uncontrolled cardiovascular disease. When using fluoroscopy for image guidance, pregnancy is also a contraindication.
Supine anterior intra-articular hip fluoroscopic procedure
The patient is placed in supine position with the involved hip slightly externally rotated. This allows the patient’s anterior musculature to relax and facilitates ease of the procedure. Prep and drape in typical sterile fashion and palpate to identify the femoral neurovascular bundle. Using antero-posterior fluoroscopic imaging, mark the skin at a spot over the center of the femoral neck. Make a skin wheal and deeper infiltration with local anesthetic with a 27- or 25-gauge needle. Once anesthetized, using a 22 gauge, 3.5 inch spinal needle, penetrate the skin and direct towards the junction of the femoral head and neck. The target may be at the center of the femoral head or lateral to the center under AP imaging (Figure 1 and 3a). Once osseous contact is attained and the needle is purchased in a firm subcapsular position, aspirate and then inject contrast to confirm intraarticular flow. The authors recommend the use of tubing when injecting both contrast and the steroid/anesthetic mixture in order to prevent inadvertent manipulation of the needle once position has been confirmed. The authors of this paper recommend injected a total volume of 8cc (3cc 1% lidocaine, 3cc 0.25% bupivacaine and 2 cc of steroid, either betamethasone (6mg/ml) or triamcinolone (40mg/ml). Occasionally, the hip capsule does not extend as distally on the femoral neck, thus the needle will need to be repositioned more proximally to obtain proper intraarticular flow patterns. Of note, using this lateral to medial approach maintains a safe distance from the femoral neurovascular bundle.
-
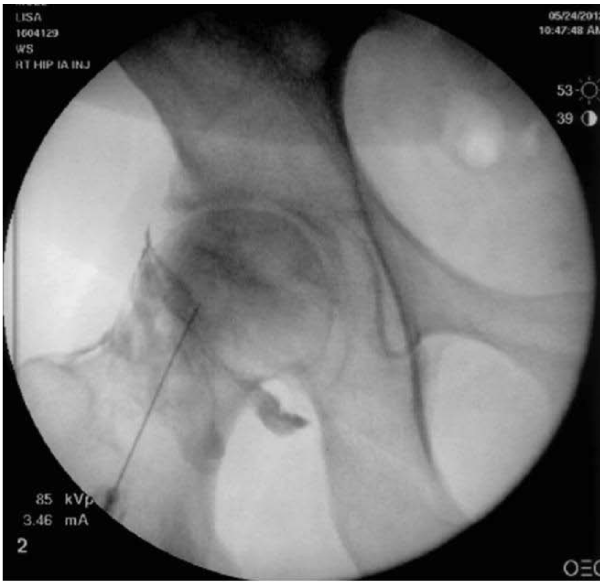
Figure 1:
Intra-articular Hip Injection- (Supine/Anterior Approach) The needle target is just lateral to the center of the femoral head. Notice the subscapsular flow of contrast extending from supero-lateral to infero-medial aspects of the capsule.
This technique is utilized in patients who are unable to lie in the lateral decubitus position. In patients with a large pannus that may cover the needle entry site, assistance to retract the pannus is necessary or implementation of a different approach should be discussed. Having the patient lie supine provides a comfortable and relaxed position for the patient.
Lateral intraarticular hip fluoroscopic procedure
Place the patient in lateral decubitus (side-lying) position with involved hip facing upward. Prep and drape in typical sterile fashion. Using both palpation and fluoroscopy, find the skin over the greater trochanter. Make skin wheal and deeper anesthesia with local anesthetic with 27- or 25-gauge needle at a spot approximately 4cm cephalad to the greater trochanter. With a 3.5 inch 22 gauge spinal needle (although a longer needle may be required in larger patients), penetrate the skin and direct towards the top of the greater trochanter under lateral imaging. Redirect the needle towards the femoral head/neck junction (Figure 2 and 3b). Of note, on lateral imaging, the smaller ipsi-lateral hip joint will be the target as the down contra-lateral hip will appear larger due to image magnification. As the target is approached, switch to an AP view and direct towards the femoral head/neck junction. Once osseous contact has been made, inject contrast to confirm intraarticular flow. The authors of this paper recommend injected a total volume of 8cc (3cc 1%$ lidocaine, 3cc 0.25% bupivacaine and 2 cc of steroid, either betamethasone (6mg/ml) or triamcinolone (40mg/ml). This approach offers some advantages in the obese patient such that a larger pannus does not need to be manipulated to provide proper needle access. In addition, a lateral entry may decrease the risk of infection and inadvertent vascular penetration.
-
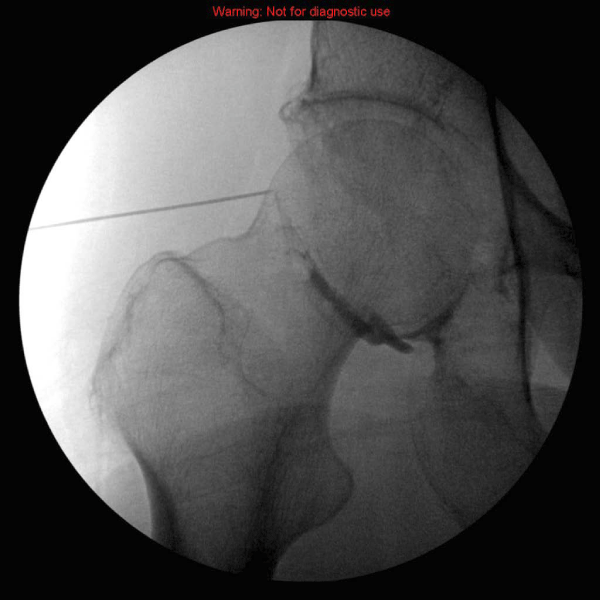
Figure 2:
Intra-articular hip injection- (Lateral Approach) The needle target is the lateral border of the femoral head neck junction. The contrast agent extends into the inferior-medial aspect of the joint and capsule.
-
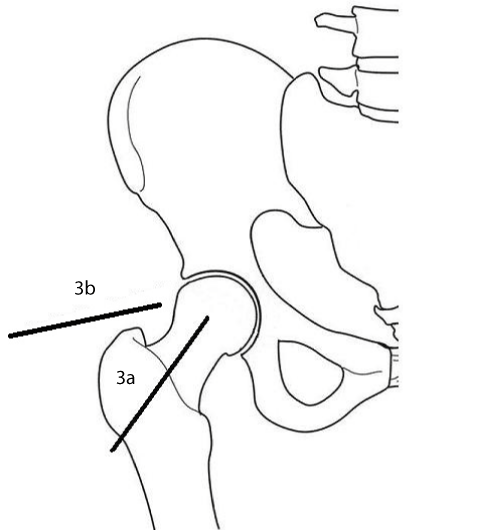
Figure 3:
Needle trajectory and target for the anterior/supine (3a) and lateral (3b) approaches.
Ultrasound guided intraarticular hip
Patient is placed in the supine position with the hip in slight internal rotation or neutral. Palpate the anterior superior iliac spine (ASIS) and orient the transducer longitudinally medial to the ASIS (Figure 4a-4b). Using a 5 MHz curvilinear transducer, visualize the anterior hip joint, femoral neck and joint capsule (Figure 4c). Turn the transducer into a transverse plane and identify the femoral vessels and ensure that they are located medially to the injection site. Color flow Doppler can further identify vascular flow. Re-orient the transducer to the long-axis view of the femoral head-neck and again using color flow Doppler to identify the lateral femoral circumflex artery to ensure it is not in the potential needle pathway. The anterior joint capsule may be approached from above or below the transducer, depending on the patient’s body habitus and possible vascular interference. It is usually easiest to map a pathway below the transducer on the anterior thigh and entering the anterior capsule in a perpendicular orientation. Once the pathway is mapped the entry point on the skin is marked with a pressure circle from a retracted ball point pen. Following the sterile prep, the skin and subcutaneous tissues are anesthetized and through this anesthetized track a syringe containing 6 mL of 1% lidocaine attached to a 3.5 inch 22 gauge spinal needle is inserted approximately 3 cm. Using sterile ultrasound gel the needle is guided toward the anterior joint capsule. The fascial planes over the iliopsoas tendon and the joint capsule are anesthetized before the needle is passed which significantly reduces discomfort. Once the capsule has been penetrated and the lidocaine is seen entering the joint space, the lidocaine syringe is removed from the needle, the syringe containing the corticosteroid is attached and the injectate is delivered. The authors of this paper recommend injected a total volume of 8cc (3cc 1% lidocaine, 3cc 0.25% bupivacaine and 2 cc of steroid, either betamethasone (6mg/ml) or triamcinolone (40mg/ml). The needle is then removed and a sterile Band-Aid is placed over the injection site.
-
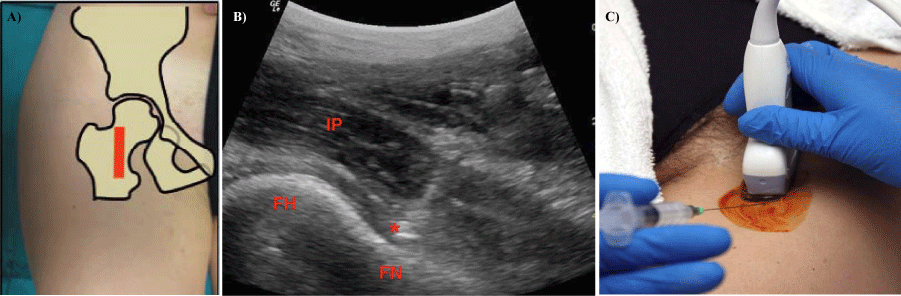
Figure 4:
4a, 4b, 4c- Ultrasound guidance of intra-articular hip injection with needle entering below the joint. (FH- femoral head, FN- femoral neck, IP- Iliopsoas muscle, asterisk- target).
Ischial Bursa Injection
Indications
Ischial bursitis is a painful condition that is primarily localized to the base of the buttock with extension to the area over the ischial tuberosity [30]. The ischial bursa is located deep to the gluteus maximus muscle and overlies the ischial tuberosity. As a result of repetitive trauma or acute damage, the bursa may become inflamed and painful. Pain in this region may also be caused by a tendinopathy at the origin of the hamstrings [31]. This is often seen in imaging studies and is related to chronic hamstring injuries. An initial therapy program consisting of anti-inflammatory medications and stretching may benefit most patients. However in those with refractory pain, a steroid injection into the ischial bursa may be performed [30].
Ischial bursa fluoroscopic procedure
The patient is placed in the prone position and fluoroscopy is used to identify bony structures of the pelvis on the affected side. The beam is centered on the obturator foramen and the target area for injection is the inferior aspect of the ischial tuberosity (Figure 5a and 5b). A 3.5 inch 22 gauge spinal needle is advanced under fluoroscopic guidance to the point of contact with the ischial tuberosity. The patient is asked to extend their hip or flex their knee to determine if the needle moves with contraction. If the needle is determined to be within musculotendinous structure, then repositioning of the needle is required. After negative aspiration, a small amount of radio opaque contrast is injected to confirm spread along the ischial bursa. Once bursogram image is obtained inject mixture of local anesthetic and/or steroid. The therapeutic medication, which includes local anesthetic (2-3 cc of either 1% lidocaine or bupivacaine 0.25%) and steroid (betamethasone (6mg/ml) or triamcinolone (40mg/ml)) is then injected with a total volume of 3 to 4 cc.
-
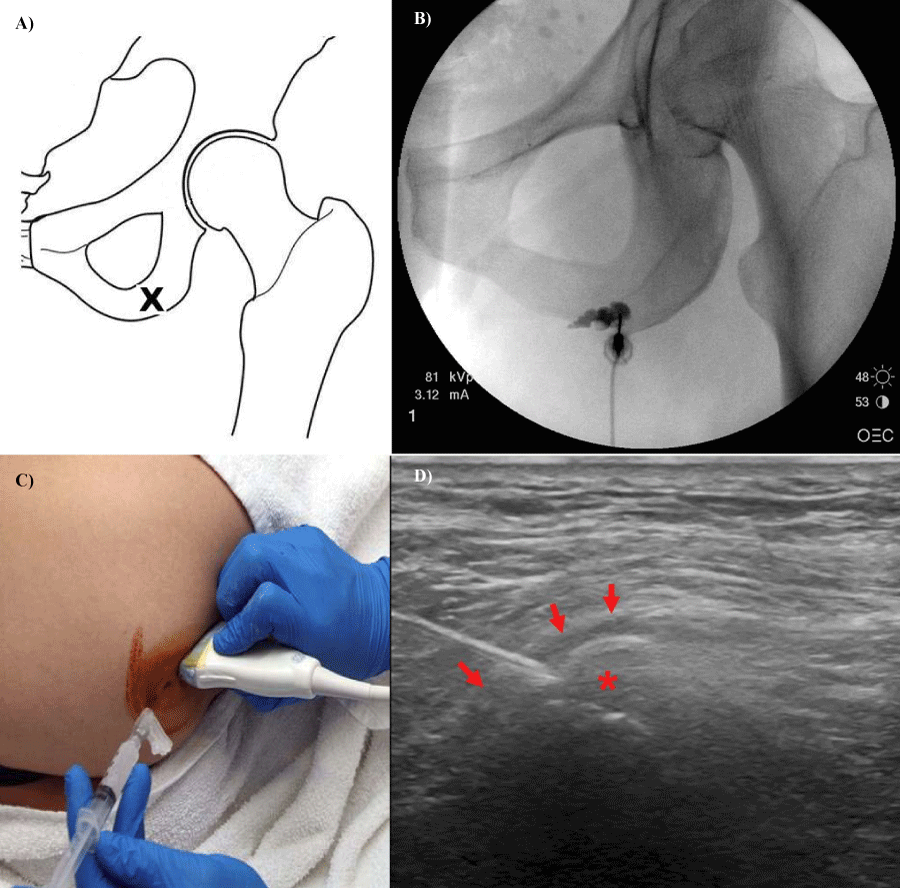
Figure 5:
5a, 5b: Target for Ischial bursa injection and contrast flow within the bursa and extending distally into the knee flexor tendons. 5c, 5d: Transducer placement for Ischial bursa injection (5c) and the needle entering the bursa (5d) along the long axis of the hamstring tendons. (Asterisk- ischial tuberosity, arrows- origin of semitendinosis).
Ultrasound guided approach to ischial bursa/hamstring origin
The patient is placed in a lateral decubitus position with the affected side up and the hips fully flexed to thin the gluteal tissues. Use either a high frequency linear transducer or a curvilinear transducer depending on the patient’s body habitus and depth of the bursa. The origins of the hamstrings as well as the ischial bursa are evaluated in both short and long axis images. When the cause of pain is isolated to either the ischial bursa or origin of the hamstrings, most commonly the semitendinosus origin, images are mapped to obtain a long axis view of the bursa and tendon origin (Figure 5c and 5d). The entry point is through the buttocks, lateral to the sciatic nerve and directly posterior to the ischial bursa. Most often at least a 10 cm spinal needle is needed to enter the target structures. The skin is prepped at the entry point of the needle and then infiltrated with local anesthesia using a 27 gauge needle. An 18 gauge introducer needle is then inserted in the same track as the smaller needle and through this introducer needle a 22 gauge spinal needle is passed under direct ultrasound visualization. The introducer needle allows precise direction of the needle without being affected by the transducer pressure which typically distorts the needle and makes visualization difficult. As the needle is advanced through the tissue planes, they are slowly infiltrated with local anesthesia until the target structure is entered. At this point the local anesthetic syringe is removed from the needle and the syringe containing the steroid mixture is placed on the syringe and injected.
Complications of ischial bursa injections may include local injection site pain and swelling. In addition if some of the anesthetic travels laterally toward the sciatic nerve, the patient may report numbness in the posterior thigh and calf regions. Rarely a patient may report transient weakness of muscles supplied by the sciatic nerve. Care must be taken to not inject into the sciatic nerve. This is avoided by minimizing or not using sedation during the injection and ensuring the patient does not report sharp, electric pain radiating into the lower extremity.
Greater Trochanter Bursa Injections
Indications
Greater trochanter pain syndrome which describes a chronic, intermittent pain and tenderness over the greater trochanter with the patient in the side-lying position, has a broad spectrum of causes including trochanteric bursitis, tendinopathies, gluteal muscle tears and external coxa saltans (snapping hip) [32]. There is an increased prevalance in older age, female gender, knee OA, obesity and low back pain patients. These may all cause altered lower limb biomechanics may be risk factors developing greater trochanteric bursitis [33,34]. It is reported to affect between 10% and 25% of the general population [35]. In addition there are three known bursa over the lateral side of hip; the greater trochanter bursa, sub gluteus medius bursa and sub gluteus minimus bursa (Figure 6). These each have distinct biomechanical implications with separate treatment approaches. Increased volumes may lead to superior gluteal nerve palsies, so it is suggested using a 2cc mixture of 1cc anesthetic and 1cc corticosteroid [36].
-
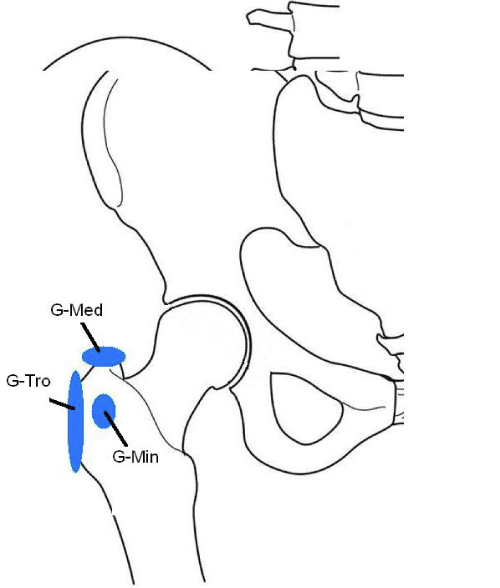
Figure 6:
Location of the three major bursa located on the lateral aspect of the hip; G-Med, sub gluteus medius; G-Tro, greater trochanteric bursa; G-min, sub gluteus minimum bursa.
Greater trochanter bursa fluoroscopic procedure
Place the patient in the lateral recumbent position with the affected side up. The hip is flexed 30 to 50 degrees and the knee is flexed 60 to 90 degrees. Using lateral fluoroscopic imaging, identify and palpate the greater trochanter over the femur from the mid-shaft proximally and mark the point of maximal tenderness or swelling. Prep and drape in usual sterile fashion. Using a 22- or 25-gauge spinal needle, the skin is penetrated perpendicular to the skin. The needle should be inserted directly down to osseous contact and then withdrawn before injecting. Rotate the beam to the true AP image and inject contrast into the bursa (Figure 7e). Once bursogram image is obtained inject mixture of local anesthetic and/or steroid. The authors of this paper recommend injected a total volume of 3-4cc (2-3 cc of 1% lidocaine or 0.25% bupivacaine and 1 cc of steroid, either betamethasone (6mg/ml) or triamcinolone (40mg/ml). Always use a 3.5 inch or longer spinal needle in order to reach the target point. Especially in larger patients, to more easily palpate and identify the greater trochanter, ask the patients to internally and externally rotate the leg. Complications include superior gluteal nerve block especially if the injectate is delivered superiorly in close proximity to the gluteus medius bursa.
-
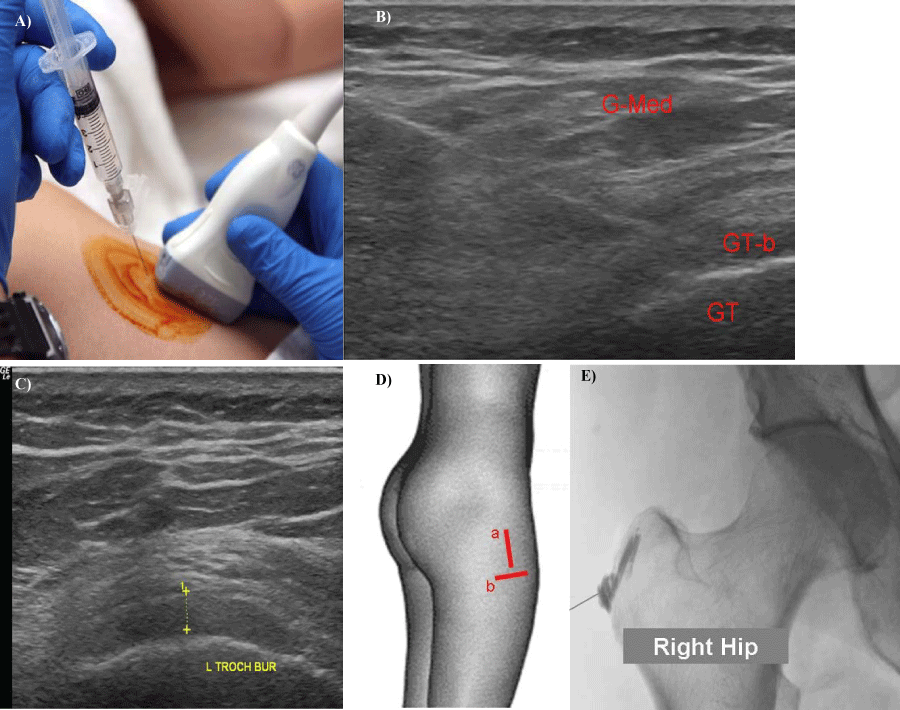
Figure 7:
7: 7a, 7b, 7c, 7d, 7e- Position of needle and (7a) transducer for (7b) ultrasound guided greater trochanteric bursa) injection. (G-med: gluteus medius muscle, GT- greater trochanter, Gt-b: greater trochanterid bursa) (7c) - Image of inflamed trochanteric bursa in short axis. (7d) - Orientation of transducer in long axis (a) and short axis (b). 7e- Fluoroscopic imaging of right greater trochanter bursogram.
Ultrasound-Guided greater trochanter bursa procedure
Place the patient in the lateral recumbent position with the affected side up. The transducer is positioned in the longitudinal axis and this injection will be performed using the in-plane approach. Using the highest frequency transducer to allow adequate depth of visualization, identify the greater trochanter, trochanteric bursa, the overlying muscles and fascia (Figure 7a). The normal bursa will measure less than 4 mm and the inflamed bursa will often be greater 10 mm in depth (Figure 7b and 7c). The pathway to bursa is then mapped and measured to ensure that a needle of adequate length is used. Commonly, a 3.5 in, 22 gauge spinal needle is required to enter this bursa. Make a skin wheal approximately 1cm distal to the end of the transducer. Penetrate the skin and advance the needle to the greater trochanter deep to the overlying muscles and tendon. Approach the greater trochanter at about a 45 degree angle to ensure that the needle is visualized. Once the needle is seen entering the trochanteric bursa and the lidocaine is seen entering the bursa, remove the lidocaine syringe from the needle and attach the one containing the corticosteroid and deliver the injectate. Again, the authors of this paper recommend injected a total volume of 3-4cc (2-3 cc of 1% lidocaine or 0.25% bupivacaine and 1 cc of steroid, either betamethasone (6mg/ml) or triamcinolone (40mg/ml). Since the needle is seen entering the bursa and flow of the injectate is seen within the bursa, contacting the greater trochanter with the needle is not necessary and the patient will have less discomfort.
Piriformis Syndrome
Piriformis myofascial pain is a potential cause of buttock and posterior leg pain which typically presents as an aching sensation over the infero-lateral gluteal region [37]. In addition, a tight piriformis muscle may cause nerve entrapment due to the anatomic association of the muscle and the sciatic nerve [37]. The piriformis muscle originates on the anterior wall of the sacrum, travels through the greater sciatic foramen and inserts on the greater trochanter of the femur [38]. The primary action of this muscle is external rotation of the femur. A constellation of symptoms consisting of buttock pain, with or without posterior thigh pain, is typically made worse with prolonged sitting [38]. When this occurs, the piriformis can become tight and focal point tenderness will reproduce these symptoms. Injection of anesthetic with or without steroid can provide a safe and effective way to offer both diagnostic and therapeutic intervention. If using only local anesthetic, 1cc of either 1% lidocaine or 0.25% bupivacaine can provide diagnostic utility. When injecting a steroid mixture, the authors suggest 1cc of local anesthetic (1% lidocaine or 0.25% bupivacaine) with 1 cc of corticosteroid (betamethasone (6mg/ml) or triamcinolone (40mg/ml)). Some recent data has advocated the use of botulinum toxin (onabotulinumtoxin A) in patients with diagnosed piriformis syndrome. In 2007, Yoon et al. [39] injected 20 patients with 50 units of botulinum toxin (onabotulinumtoxin A) using CT guidance. This group was compared to nine control patients receiving corticosteroid. Pain intensity scores were significantly lower in the botulinum group at all follow-up time-points.
Fluoroscopy - musculotendinous approach
Using fluoroscopic guidance, place the patient in prone position with anteroposterior imaging of the affected side. A true AP (without any rotation or tilt) is needed to avoid improper target localization. Visualize the femoral head within the acetabulum and mark the skin at a point corresponding with approximately the 12 o’clock position above the acetabulum (Figure 8). Prep and drape skin in normal fashion and make a skin wheal with superficial and deep infiltration of local anesthetic. Penetrate the skin using a 3.5 inch 22 gauge spinal needle in a coaxial view until bony contact of the acetabulum is attained. The needle should bounce off of bone into the surrounding piriformis muscle. Confirm proper needle placement with injection of a contrast solution, which should delineate the contour of the piriformis. The contrast flow should travel in a path from the greater trochanter towards the sacrum. Once needle placement is confirmed, inject a solution of 2cc mixture steroid and local anesthetics [40].
-
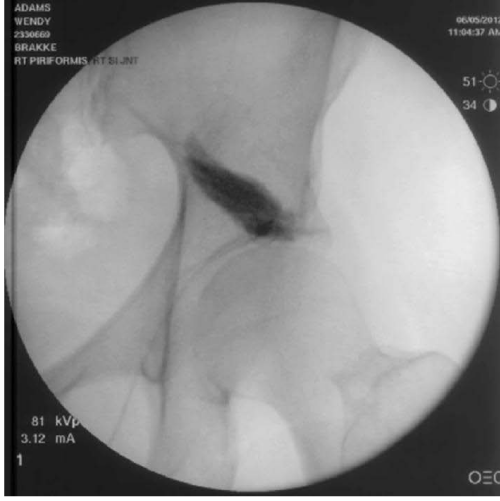
Figure 8:
Contrast pattern extending linearly on a path from the greater trochanter to the sacrum within the muscle fibers of the piriformis muscle.
Ultrasound-Guided
Using the highest frequency transducer possible to allow adequate depth of visualization, it is important to initially identify the sciatic nerve as it passes near the piriformis muscle. Using an 8 MHz linear transducer or a 5 MHz curvilinear transducer set at approximately 8-10 cm in depth; place the probe transversely between the greater trochanter and the ischial tuberosity (Figure 9a and 9b). In this position, the cross section of the sciatic nerve is identified as a honeycomb structure with mixed echogenicity and is typically oval in shape. Slowly translate the transducer probe superiorly, until the sciatic nerve courses beneath the piriformis muscle. Mark the skin lateral to the point at which the nerve and piriformis muscle intersect. Now visualize the tendon insertion onto the greater trochanter. The piriformis tendon will parallel the gluteus medius tendon and often has a hypo echoic appearance consistent with tendinopathy. If this is true then the injection will be directed toward the tendon insertion. If it is normal in appearance then position the probe along the long axis of the piriformis muscle fibers. At this point prep the skin in sterile fashion. Penetrate the skin and advance the spinal needle with continuous sonographic visualization parallel with the transducer. Once you have pierced the gluteus maximus muscle, the piriformis muscle will lie just deep to this structure. Inject mixture of local anesthetic and steroid into the piriformis muscle [41].
-
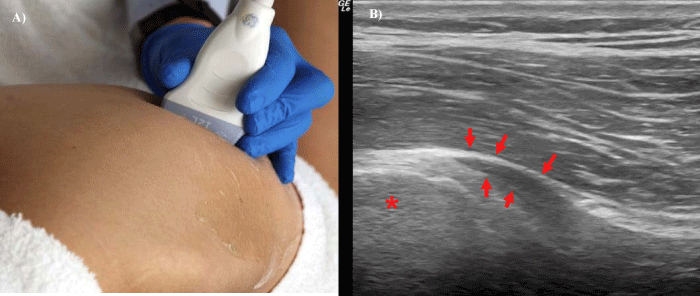
Figure 9:
9a, 9b- Transducer position in the long axis of the piriformis muscle (9a) showing insertional piriformis tendinopathy using 8MHz transducer (9b). (Asterisk- greater trochanter, arrows- insertion of piriformis muscle).
Nerve stimulator guidance (peri-sciatic approach)
Prep and drape the skin in sterile fashion. Identify the greater trochanter and posterior superior iliac spine. At a point midway between these two landmarks, make a skin wheal with superficial and deep infiltration with local anesthetic. Insert a disposable injectable monopolar needle electrode (23-25 gauge, 100 mm or greater) connected to a nerve stimulator at this point perpendicular to the skin. The nerve stimulator should be initially set to deliver 0.8 mA (2 Hz, 100µsec). The needle is advanced slowly until stimulation of the piriformis muscle is obtained. This typically occurs at a depth just superficial to bony contact. Once appropriate twitches of the piriformis muscle are elicited as evidence of thigh external rotation, 1-2 mL of local anesthetic with steroid is injected slowly with intermittent aspiration to rule out intravascular injection.
Iliopsoas Tendon/Bursa Injection
Iliopsoas tendinosis which is often seen in the setting of rheumatoid arthritis, acute trauma, overuse injury, or after total hip arthroplasty may be a cause of hip or groin pain [42,43]. Inflammation of the bursa which is between the tendon and the pelvis or anterior hip capsule can contribute to this situation. Because of the close proximity between tendon and bursa, the inflammation of one will inevitably result in inflammation of the other [44,45]. Although symptomatic medial iliopsoas snapping is rare, it may also associated with iliopsoas bursitis and tendinitis [45]. As a cause of pain after total hip arthroplasty, impingement of the iliopsoas tendon reportedly occurs in up to 4.3% of patients [46]. The iliopsoas bursa and tendon can potentially be irritated by the problems related to acetabular component such as malposition, fixation screws or extruded cement debris around anterior edge of the acetabular component [47].
Fluoroscopy - musculotendinous approach
Position the patient supine on the radiolucent table and identify the femoral neurovascular structures. Obtain AP image of the hip joint and identify the 12 o’clock position of the acetabulum (Figure 10c). This will serve as your target. Entering the skin at site directly above this target could prove more difficult in obese patients with a large pannus. In addition there is increased risk of violation of the abdominal cavity and possible bowel injury. Mark the skin at a site distal and lateral to the target over the femoral neck. Make a wheal with local anesthetic. Penetrate the skin and advance the needle using cross-beam technique with intermittent fluoroscopy until the target is reached. Inject contrast medication to ensure flow into the muscle. Once needle confirmation is obtained, inject mixture of local anesthetic and steroid. This technique has recently been described in Maher et al. [48].
-
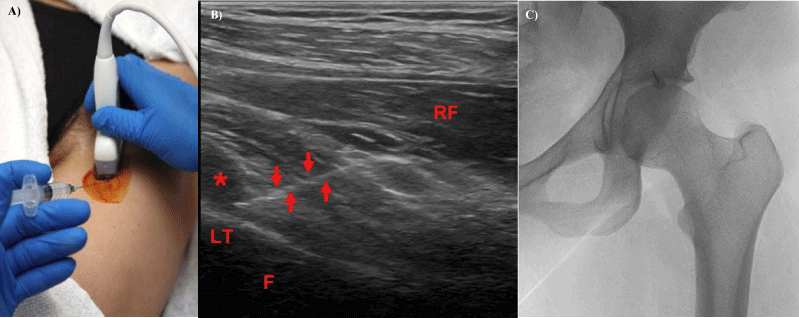
Figure 10:
10: 10a, 10b, 10c- Transducer placement (10a) and needle entering the iliopsoas bursa (10b). (Asterisk- iliopsoas bursa, LT- lesser trochanter, RF- rectus femoris, F- femur, arrows- needle targeting bursa). 10c- Fluoroscopic target for iliiopsoas injections, anterior 12o’clock position of the acetabulum.
Ultrasound-guided
Place the patient supine and examine the anterior aspect of the symptomatic hipusing transverse and sagittal planes. The iliopsoas tendon is deep to the rectus femoris muscle and lies directly over the femoral head and anterior joint capsule. The normal iliopsoas muscle/tendon has the same gray mixed echogenicy as the rectus femoris muscle and the bursa cannot be visualized. The inflammed iliopsoas is hypoechoic and distinctly different than the surrounding muscularature and the inflammed bursa is an anechoic space directly over the femoral head, situated between the joint capsule and the iliopsoas tendon. Again map the approach to the bursa in the same fashion as when the joint space was entered (Figure 10a and 10b). Prep the skin in typical sterile fashion and make a skin wheal with local anesthetic. Penetrate the skin and advancethe spinal needle under direct ultrasound guidance. When the needle is seen entering the anechoic bursa, then change the lidocaine syringe to the corticosteroid syringe and inject the bursa. Direct distention of the iliopsoas bursa can be noted and the presence of microbubbles in a peritendinous distribution can confırm the needle position.
Blocks of Sensory Branches of the Obturator and Femoral Nerves
The hip joint is innervated by the sensory articular branches of obturator, femoral and sciatic nerves [49]. There are reports of short-term hip pain relief with the obturator nerve block [50]. However, others have suggested that blocking the obturator nerve exclusively may not be sufficient for the treatment of hip pain, and that effective neural blockade of the hip joint can only be achieved by also blocking the femoral nerve [51]. CT guided, fluoroscopic and electrical stimulation guided obturator and femoral nerve blocks have been described [52,53].
Fluoroscopic approach
Place the patient in the supine position and palpate the femoral artery. Injections should be performed under fluoroscopy and sterile conditions. The sensory articular branch of obturator nerve may be blocked by inserting a needle just medial to the femoral artery below the level of the inguinal ligament under fluoroscopy (Figure 11). The needle is then advanced with intermittent imaging at the site below the inferior junction of the pubis and the ischium, which appears teardrop in shape in the antero-posterior view. In order to block the articular branch of femoral nerve, the needle is inserted from a site below the anterior superior iliac spine to the lateral aspect of the extraarticular portion of the hip joint. It is advanced to just below the anterior inferior iliac spine next to the antero-lateral margin of the hip joint. A transcutaneous electro-neurostimulator needle (21 gauge – 100 mm) may be used for sensory stimulation testing to confirm the needle position. Patients will report numbness and tingling sensation with approximately 0.5-0.7mA voltage amplitude [51]. For pulsed radio-frequency thermocoagulation, a 22-gauge (10 cm) RF cannula with 10 mm active tip can be used with the same approach as the nerve block.
-
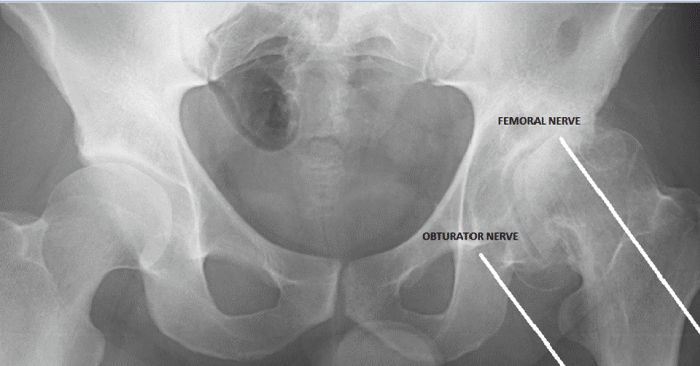
Figure 11:
Articular branches of the obturator and femoral nerves.
Conclusion
At the present time, image guided injections to diagnose and treat pathologies around the hip joint are recommended to promote safety and accuracy of interventions. Blinded injections into the deep joints such as the hip have largely been abandoned due to their failure rate and risk of complications. Intra-articular hip injections with fluoroscopy are also used safely for both diagnostic and therapeutic interventions. In addition, ultrasonography, while largely operator dependent, has come into favor especially for soft tissue injections around hip. This is due in part to the ability to visualize soft tissue structures and provide real time dynamic assessment of various structures.
Whatever the modality (fluoroscopy or ultrasound), the diganosis of hip and pelvic pathologies must be centered on a thorough history of physical examination. Targeted interventions may provide additional imformation and should be utilized in order to treat pain and restore functional mobility.
-
-
- Waddell G, Feder G, McIntosh A, Lewis M, Hutchinson A (1996) Low back pain: clinical guidelines and evidence review. London: Royal College of General Practitioners.
- Croft PR, Macfarlane GJ, Papageorgiou AC, Thomas E, Silman AJ (1998) Outcome of low back pain in general practice: a prospective study. BMJ 316: 1356–1359.
- Frost H, Lamb SE, Shackleton C (2000) Functional restoration programme for chronic low back pain. A prospective outcome study. Physiotherapy 86: 285-293.
- Hay E, Mullis R, Lewis M, Vohora, K (2005) Comparison of physical treatments versus a brief pain-management programme for back pain in primary care: a randomised clinical trial in physiotherapy practice. Lancet 365: 2024-2030.
- Critchley DJM, Ratcliffe JP, Noonan SB, Jones RHF, Hurley MVP (2007) Effectiveness and Cost-Effectiveness of three types of physiotherapy used to reduce chronic low back pain disability: A pragmatic randomized trial with economic evaluation. Spine 32: 1474-1481.
- Ewert T, Limm H, Wessels T, Rackwitz B, Von Garnier K, et al. (2009) The comparative effectiveness of a multimodal program versus exercise alone for the secondary prevention of chronic low back pain and disability. PMR 1: 798-808.
- Lamb SE, Hansen Z, Lall R, Castelnuovo E, Nichols V (2010) Potter R, Underwood MR. Group cognitive behavioural treatment for low-back pain in primary care: a randomised controlled trial and cost-effectiveness analysis. Lancet 375: 916-923.
- NICE Low Back Pain – Early Management of Persistent Non-specific Low Back Pain.
- Van Geen J, Edelaar M, Janssen M, van Eijk J (2007) The long term effect of multidisciplinary back training: a systematic review. Spine 32: 249-255.
- Richards MC, Ford JJ, Slater SL, Hahne AJ, Surkitt LD, et al. (2013) The effectiveness of physiotherapy functional restoration for post-acute low back pain: a systematic review. Man Ther 18: 4-25.
- Fairbank J, Frost H, Wilson-MacDonald J, Yu LM, Barker K, et al. (2005) Randomised controlled trial of compare surgical stabilisation of the lumbar spine with an intensive rehabilitation programme for patients with chronic low back pain. BMJ 330: 1233.
- Mannion AF, Brox JI, Fairbank JCT (2013) Comparison of spinal fusion and non operative treatment in patients with chronic low back pain: long term follow-up of three randomized controlled trials. Spine J 13: 1438-1448.
- Dagenais S, Tricco AC, Haldeman S (2010) Synthesis of recommendations for the assessment and management of low back pain from recent clinical practice guidelines. Spine J 10: 514-529.
- Airaksinen O, Brox JL, Cedraschi C, Hildebrandt J, Klaber-Moffett J, et al. (2006) Chapter 4 European guidelines for the management of chronic low back pain in primary care. Eur Spine J 15: 5192-5300.
- Chou R, Qaseem A, Snow V, Casey D, Cross JT jr, et al. (2007) Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med 147: 478-491.
- Smeets RJEM, Vlaeyen JWS, Hidding A, Kester ADM, van der Heijden GJMG, et al. (2006) Active rehabilitation for chronic low back pain: Cognitive-behavioral, physical or both? First direct post-treatment results from a randomized controlled trial. BMC Musculoskelet Disord 7: 5.
- Van Middelkoop M, Rubinstein SM, Kuijpers T, Verhagen AP, Ostelo R, et al. (2011) A systematic review on the effectiveness of physical and rehabilitation interventions for chronic non-specific low back pain. Eur Spine J 20: 19-39.
- Zigmund AS, Snaith RP (1983) The Hospital Anxiety and Depression Scale. Acta Psychiatra Scand 67: 361-370.
- Fairbank J, Pynsent P (2000) The Oswestry Disability Index. Spine 25: 2940-2953.
- Jensen MP, Karoly P, Braver S (1986) The measurement of clinical pain intensity: a comparison of six methods. Pain 27: 117-126.
- Miller RP, Kori S, Todd D (1991) The Tampa Scale: a measure of kinesiophobia. Clin J Pain 7: 51–52.
- Sullivan MJL, Bishop SC, Pivik J (1995) The Pain Catastrophizing Scale: Development and validation. Psychological Assessment 7: 524-532.
- Chapman JR, Norveu DC, Hermsmeyer JT, Bransford BJ, Devine J, et al. (2011) Evaluating common outcomes for measuring treatment success for chronic low back pain. Spine 36: S54-68.
- Nicholas MK (2007) The pain self-efficacy questionnaire: Taking pain into account. Eur J Pain 11: 153-163.
- Miles CL, Pincus T, Carnes D, Taylor SJC, Underwood M (2011) Measuring pain self efficacy. Clin J Pain 27: 461-470.
- Durlak JA (2009) How to select, calculate and interpret effect sizes. J Pediatr Psychol 34: 917-928.
- Guzman J, Esmail R, Karjalainen K, Malmivaara A, Irwin E, et al. (2001) Multidisciplinary rehabilitation for chronic low back pain: systematic review. BMJ 322: 1511-1516.
- Flor H, Fydrich T, Turk DC (1992) Efficacy of multidisciplinary pain treatment centers: a meta-analytic review. Pain 49: 221-230.
- Waterschoot FPC, Dijkstra PU, Hollak N, de Vries HJ, Geertzen JHB (2014) Dose or content? Effectiveness of pain rehabilitation programs for patients with chronic low back pain: A systematic review. Pain 155: 179-189.
- Williams AC (2014) How do we understand dose of rehabilitative treatment? Pain 155: 8-9.
- Gatchel RJ, Mayer TG (2008) Evidence informed management of chronic low back pain with functional restoration. Spine J 8: 65-69.
- Dopson S, Fitzgerald L, Ferlie E, Gabbay J, Locock L (2002) No magic targets! Changing clinical practice to become more evidence based. Health Care Manage Rev 27: 35-47.
- Moore AP, Jull G (2012) Research culture development in the workplace – the key to professional success. Man Ther 17: 487-488.
- Rycroft-Malone J, Wilkinson JE, Burton CR, Andrews G, Ariss S, et al. (2011) Implementing health research through academic and clinical partnerships: a realistic evaluation of the Collaborations for Leadership in Applied Health Research and Care (CLAHRC). Implement Sci 6: 74.
-
-
-
-
-
-
Peertechz is an international Open Access, peer-reviewed podium which aims to provide scientists, researchers, academicians...
Submit Manuscript -
-









Table 1:
Table 1: